

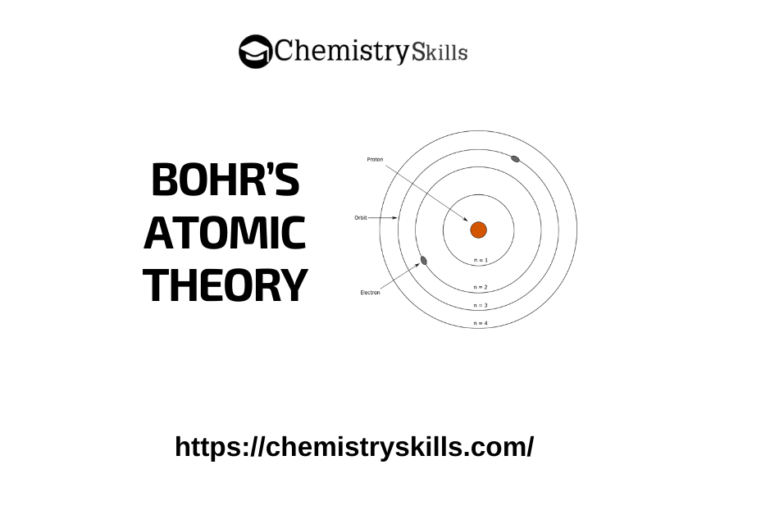
Furthermore, the radiation predicted by the Rutherford model would have been a continuous spectrum of every color – in essence white light that when passed through a prism would display all of the colors of the rainbow (Figure 1). However, for the most part, atoms are stable, lasting literally billions of years. Unfortunately, this same system predicted that electrons orbiting in the manner that Rutherford described would lose energy, give off radiation, and ultimately crash into the nucleus and destroy the atom.

Rutherford’s planetary model of the atom was based upon classical physics – a system that deals with physical particles, force, and momentum. While Rutherford’s model explained many observations accurately, it was found to have flaws.
Niels bohr atomic theory series#
Like so many before him, Bohr built upon the work of his predecessors, and for Bohr, part of that foundation had been built by Ernest Rutherford.īased upon a series of experiments, Rutherford proposed the planetary model of the atom in which electrons swirled around a hard, dense nucleus (see Atomic Theory I: The Early Days). The most intense period of progress took place between the late 19 th and early 20 th century, and it hinged heavily on the work of a Danish physicist named Niels Bohr. But that was all about to change, and quite dramatically. Given that many centuries had elapsed between the earliest ideas of the atom and Dalton’s work, it would be fair to say that the evolution of atomic theory had been a gradual one, with progression in the field being steady rather than spectacular. French chemists Antoine Lavoisier and Joseph Proust, with their Law of Conservation of Mass in 1789 and Law of Definite Proportions in 1799, respectively, each laid the groundwork for Englishman John Dalton’s work on the Law of Multiple Proportions (Dalton, 1803). The story of atomic theory first encounters reproducible, scientific (evidence based) proof in the late 18th century. In fact, one could argue that the history, struggle, and achievement that is threaded through the development of understanding matter at the atomic level is the quintessential story of the scientific method. This gradual, logical progression, where atomic structure evolved from being a simple, philosophical idea, through to the ultra-sophisticated world of the Higgs boson particle discovered in the early part of the 21 st century, represents a wonderful example of the evolution of a scientific idea, and the application of the scientific process. (You can read more about this is in our modules Early Ideas about Matter: From Democritus to Dalton and Atomic Theory I: The Early Days.) Despite the slow pace, it is crucial to understand that the process was a methodical one as each scientist built upon earlier ideas. Starting with the ancient Greeks, and moving through to the beginning of the 19th century, the story unfolds relatively slowly. The earliest ideas about matter at the atomic level were built over many centuries.

Understanding Scientific Journals and Articles.Using Graphs and Visual Data in Science.


 0 kommentar(er)
0 kommentar(er)
